Animal Laboratory Design and Management Plan - Nanjing Expansion Technology [Free Consultation]
Release time:
2025-06-24 10:15
System Deconstruction of This Document Animal Laboratory This document details a complete lifecycle construction system for animal laboratories, deeply integrating GB14925-2010 national standards and practical specifications. By analyzing the three-level microbial control system of ordinary, barrier, and isolation environments, combined with core elements such as site selection, airflow organization, and equipment configuration, it comprehensively covers the technical details of animal laboratories from planning and design to daily operation and maintenance. It provides research institutions with a construction guide that is both professional and practical, helping to improve the research support efficiency and compliance management level of animal laboratories.
For Nanjing Expansion Technology Animal Room case studies, please click to view → Peking University Institute of Molecular Medicine, Nanjing Translational Research Institute
I. Multidimensional Analysis of the Internal and External Environments of Animal Laboratories
1.1 Internal Environment: The Micro-Ecosystem of Animal Survival
The internal environment of an animal laboratory includes two dimensions: "macro-environment" and "micro-environment":
- Macro-environment Refers to the laboratory space where the animal cages are located. It needs to meet the air exchange requirements of 10-20 times/h for barrier environments and 20-50 times/h for isolation environments. Air cleanliness of 10,000 grade (barrier) to 100 grade (isolation) is achieved through a three-stage filtration system (primary, medium, and high efficiency filters with efficiencies of G4, F8, and H14 respectively).
- Micro-environment Focuses on the micro-ecology within the cage, such as SPF-grade rat cages, which need to control temperature at 20-26℃, daily temperature difference ≤4℃, relative humidity 40-70%, air velocity at 0.1-0.2m/s, ammonia concentration ≤14mg/m³, noise ≤60dB, and lighting following a 12h light/12h dark diurnal rhythm.
Case Study A barrier environment animal laboratory at a certain university uses an IVC (Independent Ventilated Cage) system. Each mouse cage is equipped with a separate supply and exhaust air channel, and the air cleanliness inside the cage reaches 100 grade, with 0 colony-forming units/plate, effectively solving the problem of cross-contamination in traditional barrier environments.
1.2 External Environment: Macro-Control Factors for Facility Site Selection
The planning of the external environment of an animal laboratory should follow the "three avoidances and three separations" principle:
- Avoidance of pollution sources Stay away from industrial storage yards that emit dust (such as cement silo areas) and chemical plants that emit harmful gases (the upwind side of the minimum annual frequency wind direction should maintain a distance of more than 500 meters);
- Avoidance of transportation hubs Maintain a distance of more than 500 meters from railways and airport runways, and a distance of ≥300 meters from main city roads. Measured data show that the noise level at 100 meters from the highway can be reduced to below 55dB;
- Separation from living areas Maintain a sanitary protection distance of ≥50 meters from employee dormitories, canteens, and other living areas, and locate it on the leeward side of the prevailing wind direction in summer.
II. Technical Architecture and Operation Management of Three-Level Environmental Facilities
2.1 Ordinary Environment: Functional Implementation Plan for Basic Breeding
2.1.1 Building Layout and Airflow Organization
A single corridor (mixed corridor) layout is adopted, with a corridor width of 1.5 meters and a door width of 1.0 meter. The hazardous area needs to have an independent negative pressure room (pressure ≤-10Pa). The ventilation system can use natural ventilation combined with mechanical exhaust, with an air exchange rate of 8-10 times/h. Rat-proof nets (aperture ≤1cm) should be installed on the exhaust vents.
2.1.2 Daily Management Regulations
- Personnel Access Before entering, change into cotton work clothes and rubber shoes, wear disposable work hats and masks, and wash hands with a 0.5% peracetic acid solution;
- Item Handling Feed is dried at 60℃ for 4 hours, bedding uses pine shavings (dust content ≤5%), and is sterilized at 121℃ for 30 minutes;
- Disinfection Process 84 disinfectant (1:100 dilution) is used weekly to wipe the cages, the floor is mopped with a 2% glutaraldehyde solution, and ultraviolet light disinfection is performed twice daily for 60 minutes each time.
2.2 Barrier Environment: The Core Carrier of SPF-Grade Animal Experiments
2.2.1 Design Concept and Pressure Difference Control
A "double corridor + buffer room" layout is adopted, and the pressure gradient strictly follows:
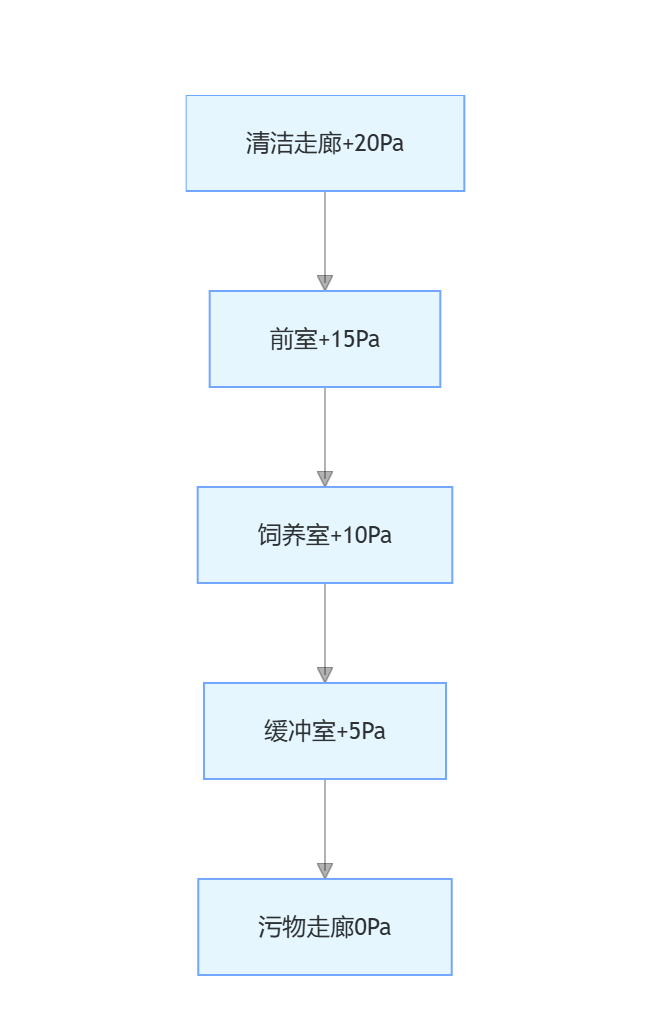
The air filtration system uses fan filter units (FFU). Primary filters are cleaned every 15 days (replaced when the pressure drop exceeds 50% of the initial value), medium efficiency filters are replaced every 3 months, and high efficiency filters are tested for leakage rate annually (PAO method), and replaced when the air velocity drops to 80% of the design value.
2.2.2 Standard Procedures for Personnel and Material Flow
- Personnel Path :
Preparation room → First change (change into ordinary work clothes) → Shower → Second change (wear sterile clothing) → Air shower (30 seconds, air velocity ≥25m/s) → Clean corridor → Breeding room → Dirty corridor → Washing and disinfection room → External area - Item Sterilization :
Feed is packaged in double-layer polyethylene bags and irradiated with cobalt 60 (dose 25kGy); cages are soaked in 0.1% benzalkonium chloride solution for 30 minutes, then treated in a 134℃ pre-vacuum sterilizer for 45 minutes; surgical instruments are sterilized using low-temperature plasma sterilization (hydrogen peroxide concentration 6mg/L, sterilization temperature 55℃).
2.3 Isolation Environment: The Ultimate Barrier System for Germ-Free Animals
2.3.1 Core Equipment and Technical Parameters
- Isolator Made of 15-micron polyethylene film, with a volume ≥0.5m³, equipped with a nitrile rubber glove (thickness 0.3mm), and glove airtightness test pressure ≥200Pa;
- Ventilation System Air dust particles (≥0.5μm) ≤3.5 per liter after high-efficiency filter (H14 grade), air exchange rate 15-20 times/h, maintaining positive pressure 100-150Pa;
- Sterilization Chamber Using vaporized hydrogen peroxide sterilization (concentration 1000ppm, acting time 30 minutes), sterilization rate of biological indicator (Geobacillus stearothermophilus) ≥10^6 after sterilization.
2.3.2 Special Operation Specifications
- Cesarean Section Introduction After wiping the mother mouse with 70% alcohol, place it in the sterilization chamber (0.5% povidone-iodine solution), perform a cesarean section in the isolator, and transfer the pups to a sterile rearing box;
- Item Transfer Feed needs to be sterilized twice under high pressure (first time 121℃/60 minutes, second time 134℃/30 minutes), and surface disinfection is performed using a transfer chamber (double-door design) outside the isolator before transfer;
- Environmental Monitoring Collect air samples inside the isolator weekly (impact method), and the number of colonies should be undetectable after 48 hours of culture. Conduct mycoplasma detection (PCR method) monthly.
III. Standardized Construction System of Facilities and Buildings
3.1 Site Selection and Building Material Standards
3.1.1 Environmental Risk Assessment Indicators
| Pollutant Source Type | Safety Distance | Control Indicators |
|---|---|---|
| Industrial Dust Emission Source | ≥500m | Daily average PM10 concentration ≤0.3mg/m³ |
| Traffic Noise Source | ≥300m | Daytime noise ≤55dB, nighttime ≤45dB |
| Medical Institution | ≥1000m | Sampling 500m downstream from the wastewater outlet |
3.1.2 Technical Requirements for Building Materials
- Walls Using stainless steel plates (304 material, thickness ≥1.2mm) or epoxy resin coating (dry film thickness ≥0.5mm), with a radius of curvature of the inner and outer corners ≥50mm;
- Floor Barrier environment uses PVC rolls (seam welding treatment), isolation environment uses epoxy resin self-leveling (compressive strength ≥70MPa), slope 1:100 towards floor drain;
- Ceiling Using aluminum alloy keel + color steel plate (thickness ≥0.426mm), hanging parts need to be treated for corrosion prevention, and all gaps are filled with silicone sealant.
3.2 Refined Layout of Functional Areas
3.2.1 Reference for Area Ratio of Functional Areas
| Functional Area | Proportion of Total Facility Area | Core Configuration |
|---|---|---|
| Production Area | 35-40% | Breeding room (≥20m²), expansion breeding room |
| Experimental Area | 30-35% | Operating room (≥15m²), observation room |
| Auxiliary Area | 25-30% | Washing and disinfection room (≥30m²), waste treatment room |
| Front Area | 5-10% | Office, feed storage room |
3.2.2 Construction Points for Special Areas
- Isolation and Quarantine Room Independent air conditioning system, equipped with a biosafety cabinet (BSC-II grade), maintaining a distance of ≥10 meters from the breeding area, and setting a double-door interlocked pass-through window;
- Washing and Disinfection Room Barrier environment needs to be equipped with a double-door high-pressure sterilizer (volume ≥1.5m³), ordinary environment can use a chemical disinfection tank (effective chlorine concentration 1000mg/L);
- Radioactive Laboratory Lead plates are applied to the walls (thickness ≥2mm), a radioactive wastewater collection pool is set on the ground (volume designed as 1.5 times the daily discharge), and exhaust gas needs to be filtered through activated carbon (residence time ≥1 second).
IV. Full Process Management and Implementation of National Standards
4.1 Full Life Cycle Management of Experimental Animals
4.1.1 Procurement and Transportation Standards
- Supplier Audit Need to provide "Experimental Animal Production License", microbial detection report of the past 3 months (SPF grade animals need to include 18 items of pathogenic microorganism detection);
- Transportation Conditions Refrigerated trucks are used in summer (temperature controlled at 20-26℃), and automatic drinking water devices need to be equipped if transportation time exceeds 6 hours. Different strains of animals need to be transported in separate cages (e.g., C57BL/6 and BALB/c need to be separated by ≥50cm).
4.1.2 Quarantine and Health Monitoring
- Isolation Observation Newly introduced animals need to be observed in the quarantine room for 14 days, with daily records of body temperature (normal body temperature for rats is 37.5-38.5℃), food and water intake;
- Microbiological Detection Sentinel animal detection is conducted quarterly for SPF-grade animals (selecting 5% of the population), with detection items including Salmonella, Sendai virus, and 20 other indicators.
4.2 Item and Environmental Monitoring System
4.2.1 Verification of Item Sterilization Effectiveness
- Physical Monitoring High-pressure sterilization requires recording temperature (121℃ or 134℃), pressure (103.4kPa or 210kPa), and time (30-45 minutes);
- Chemical Monitoring Use 3M pressure steam sterilization indicator tape (turns dark brown at 121℃, black at 134℃), paste one strip on each package;
- Biological Monitoring Use Geobacillus stearothermophilus biological indicator (ATCC7953) monthly. After sterilization, culture for 48 hours. The positive control group should show full growth, while the experimental group should show sterile growth.
4.2.2 Dynamic Monitoring of Environmental Indicators
| Indicator Type | Monitoring Frequency | Detection Method | Standard Limit Value |
|---|---|---|---|
| Temperature and Humidity | Real-time Monitoring | Temperature and humidity sensor (accuracy ±0.5℃/±3%) | 20-26℃/40-70% |
| Pressure Difference | Real-time Monitoring | Pressure difference gauge (accuracy ±1Pa) | Barrier + 20-50Pa, Isolation + 100-150Pa |
| Dust Particles | Once per quarter | Laser dust particle counter | Barrier 10,000 grade (≥0.5μm≤352000/m³) |
| Settling Bacteria | Once per month | 9cm Petri dish exposed for 30 minutes | Barrier ≤3/dish, Isolation no detection |
V. Advanced Design of Special Animal Laboratories
5.1 Infectious Animal Laboratory (ABSL)
5.1.1 Negative Pressure Gradient Design
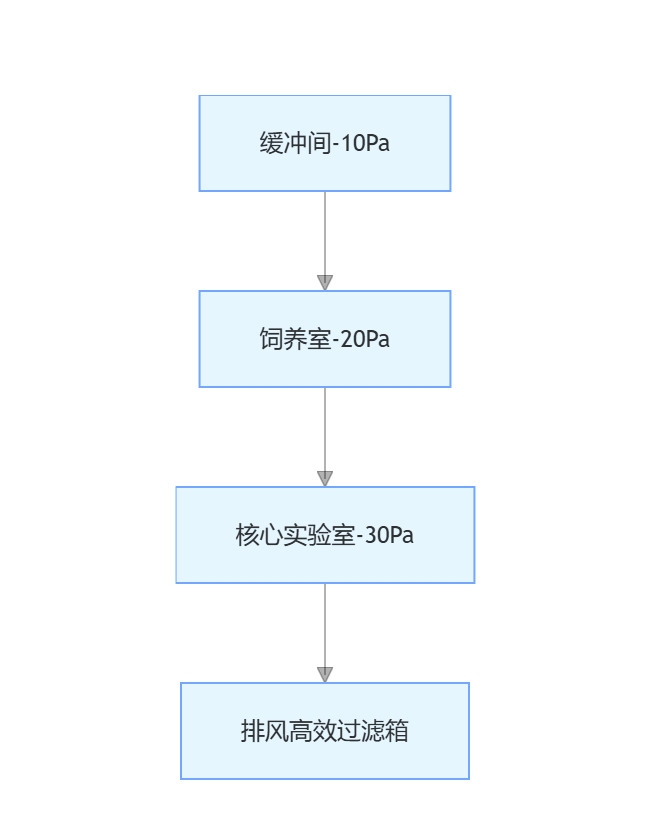
Exhaust air needs to go through two stages of high-efficiency filtration (H14 grade), the exhaust port is 3 meters above the roof, with no building air intakes within 20 meters, the indoor airflow organization adopts top-supply bottom-exhaust, air supply speed 0.35m/s, exhaust speed 0.5m/s.
5.1.2 Biosafety Measures
- Wastewater Treatment Set up a high-temperature sterilization pool (93℃ maintained for 30 minutes), or use chlorine disinfectant (residual chlorine ≥50mg/L, contact time ≥1 hour);
- Waste Treatment Infectious bedding needs to be double-layered in yellow medical waste bags, sterilized at 134℃ for 60 minutes before incineration. Incinerator exhaust gas blackness ≤ grade 1, dioxins ≤0.5ngTEQ/m³.
5.2 Radioactive Animal Laboratory
5.2.1 Shielding Design Calculation
According to the activity of the radioisotope (such as 18F-FDG), use the lead shielding thickness calculation formula:
x = \frac{\ln(N_0/N)}{\mu}
Where x is the lead thickness (cm), μ is the linear attenuation coefficient (cm⁻¹) of lead for the energy γ ray, N₀/N is the ratio of dose rate before and after shielding. For example, when 18F (γ energy 0.511MeV) needs to reach a dose limit of 1mSv/h, the lead shielding thickness is about 2.5cm.
5.2.2 Three Waste Treatment Standards
- Waste Gas After activated carbon filtration (iodine adsorption value ≥800mg/g), a radioactivity monitoring instrument (threshold 0.2μSv/h) is set at the discharge port;
- Wastewater Set up a three-stage decay pool (total volume designed according to 10 times the daily discharge), after 10 half-lives of decay, total α≤1Bq/L, total β≤10Bq/L can be discharged;
- Solid Waste Divided into degradable (such as paper) and non-degradable (such as syringes), placed in special containers respectively. When the surface contamination level α≤0.04Bq/cm², β≤0.4Bq/cm², it can be treated as ordinary waste.
Appendix: Quick Reference Table of Key Technical Parameters
| Item | Ordinary Environment | Barrier Environment | Isolation Environment |
|---|---|---|---|
| Air Cleanliness | Non-clean | 10,000 grade (ISO7) | 100 grade (ISO5) |
| Pressure Gradient (Pa) | No requirement (toxic area -10) | +20~+50 | +100~+150 |
| Ventilation rate (times/h) | 8-10 | 10-20 | 20-50 |
| Feed Sterilization Method | Drying | Cobalt 60 irradiation | Two high-pressure sterilizations |
| Personnel Access Procedure | Changing clothes + Hand washing | Shower + Air shower | Isolator glove operation |
| Falling bacteria (cfu/dish) | ≤30 | ≤3 | Not detected |
(Note: All technical indicators in the full text are based on GB14925-2010 "Laboratory animals Environment and facilities" and GB19489-2008 "General requirements for laboratory biosafety".)
Related News



